
The structure that results upon correctly executing this pairing process is shown below. Because the dots on an electron dot structure can be placed on any "side" of the elemental symbol, this rotation changes the orientation of the structure, but does not alter its meaning. When executing this pairing step, the electron dot structure for one of the atoms should be rotated so that its unpaired electron aligns with the unpaired electron on the other atom. Therefore, in order to satisfy the valences of each of these elements, hydrogen's unpaired electron must be paired with iodine's unpaired electron, in order to create a shared pair of electrons. As a result, neither can be designated as the central atom. However, in the current example, hydrogen and iodine have the same number of unpaired electrons. Typically, the element with more unpaired electrons becomes the central atom in a Lewis structure, and the other element is used as the surrounding atom. A chemically-correct electron dot structure for each of these elements is shown below.īased on the structures shown above, hydrogen has 1 unpaired electron, as does iodine.
.jpg)
Iodine, which is located in Group 7A, has 7 valence electrons.

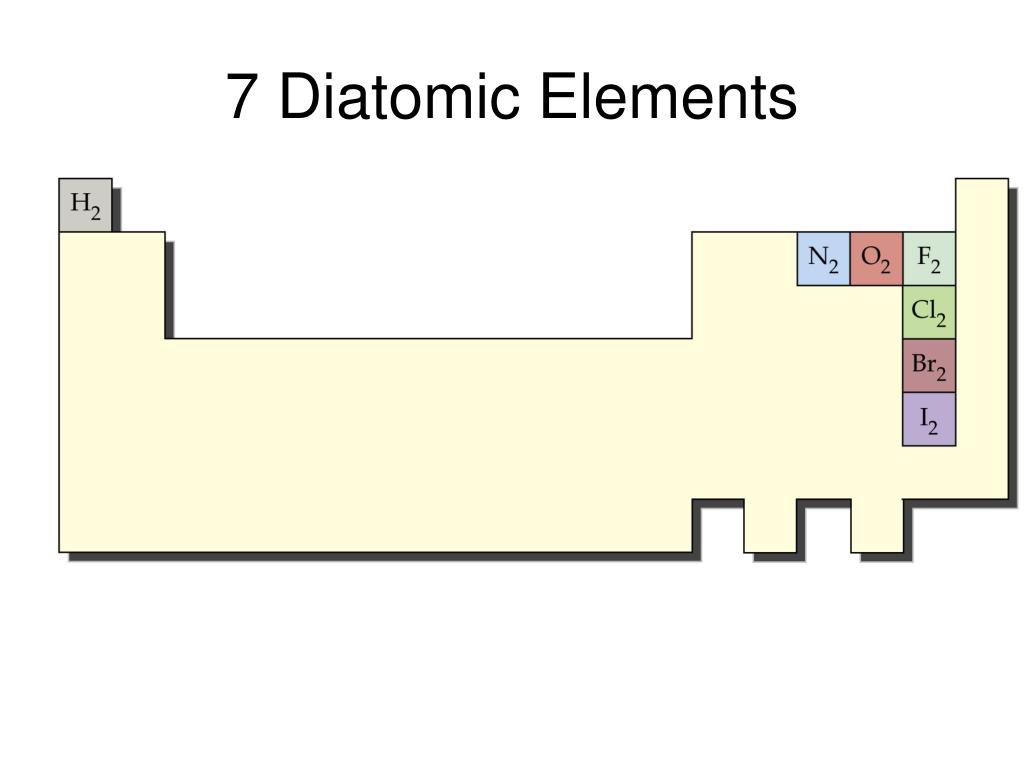
Since hydrogen is found in Group 1A of the periodic table, it contains 1 valence electron. As before, the resultant structures will be used as the basis for deriving the chemical formulas and chemical names of the corresponding covalent molecules.ĭrawing Lewis Structures of Diatomic MoleculesĪ modified version of the procedure that was presented in Section 3.15 can be used to draw the Lewis structures of both homonuclear and heteronuclear diatomic molecules, as described below.įor example, consider hydrogen and iodine.īased on the combinations listed in Section 3.14, hydrogen and iodine, which are both non-metals, will combine to form a covalent molecule. The following paragraphs will detail a modified process for drawing Lewis structures that can be applied to generate homonuclear and heteronuclear diatomic molecules. These diatomic molecules can be classified as either homonuclear, meaning that they contain two atoms of the same element, or heteronuclear, which requires that they be comprised of one atom of two different elements. However, some covalent molecules consist of only two atoms, in total, and, therefore, have no true central atom.

Because the central atoms in all of the previous examples had more than one unpaired valence electron, multiple surrounding atoms were used in the corresponding pairing processes. One surrounding atom was paired with each of the central atom's unpaired electrons, in order to create a shared pair of electrons. Specifically, the element with more unpaired electrons became the central atom, and the other element was used as a surrounding atom. In every example that was presented, the number of unpaired electrons contained within both of the given elements was determined, in order to assign the relative placement of these elements within the final covalent molecule. These two-dimensional pictures were then used as the basis for deriving the corresponding chemical formulas and chemical names for a variety of covalent molecules. The previous sections have presented and applied the process for drawing the Lewis structures of covalent molecules. Write the chemical formulas and chemical names of homonuclear and heteronuclear diatomic molecules.Draw Lewis structures of homonuclear and heteronuclear diatomic molecules.Distinguish between homonuclear and heteronuclear diatomic molecules.


 0 kommentar(er)
0 kommentar(er)
